The humble pine cone found in the forests across the world holds the seed of the new. But there is another pine cone we must consider, a cone perhaps mocked in the title of a drug we must question, welcome to clozapine.
The cone of which I speak is the third-eye of esoteric knowledge while to the medical world it is named the pineal gland. The Catholic Church is built upon its importance and symbolised in all the regalia and even in stone. Above is the coat of arms for the pope as the holder of the keys to heaven as we witness it through our third eye, under the guidance of the church doctrine and the dogma to help those who are apt to fall from the path, or so we have been taught. The keys are below the pine cone, with its two arteries coming out from its centre, splayed to the left and right to show the essence of heavens connection comes through the cone.
The pineal gland is important to the church as the gateway or portal to the heaven she represents, the arbitrator of the covenant between god and man through the message brought in Christ, with Jesus’s journey representing a picture of the covenant itself.
If the Pineal Gland is the key to human metaphysical understanding, or, our connection to the universe, then we need to fully understand how this gland can be affected by the pharmaceutical corporations.
The National Institute for Health and Clinical Excellence (NICE) published guidelines in 2009 on the treatment of schizophrenia, which affects about one in every 100 people in Britain. The watchdog recommended that oral anti-psychotic medications should be offered to people with newly diagnosed schizophrenia, although patients should take clozapine only after trying at least two other drugs.
source
Nice today is a big part of the Department for Work and Pensions work programmes and all things social-medical and service, its actions must be known and in full public view, we need to look at what they are pushing in their field, so let us take a look at Clozapine or perhaps more correctly the ability to close-a pine, a hideous chemical cocktail dished out by doctors and psychiatrist’s.
NICE are also heavily involved in the agenda called Behaviour Change, straight out of the Constitution Unit and House of Lords. But the real shocker shows itself when you consider this agenda includes behaviour medicine, and it is in the schools, it is to be found governing local authorities, in fact we are speaking of a subject that is at the core of the realm of the corporate new world.
Clozapine (sold as Clozaril, Gen-Clozapine in Canada, Azaleptin, Leponex, Fazaclo, Froidir; Denzapine, Zaponex in the UK; Klozapol in Poland, Clopine in Australia and New Zealand)
 Clozapine began life in 1958 and was handed to the world’s leading psychopharmacologist Pierre Deniker to assess its value. At the time the neuroleptic/antipsychotic group of drugs was regarded as very safe, yet when Several of Deniker’s patients died on Clozapine and, startled by the number and range of deaths he said it was Evident that it should not be developed.
Clozapine began life in 1958 and was handed to the world’s leading psychopharmacologist Pierre Deniker to assess its value. At the time the neuroleptic/antipsychotic group of drugs was regarded as very safe, yet when Several of Deniker’s patients died on Clozapine and, startled by the number and range of deaths he said it was Evident that it should not be developed.
The company that made Clozapine (Wander) paid no heed to his findings and qualified conclusion, because of course business and clinical evidence are two completely different monsters.
The development of Clozapine continued after Wander was taken over by Sandoz and perhaps the reason Sandoz purchased the company. In 1975 there was a series of deaths of people while on Clozapine following a severe drop in white blood cell counts to patients in Finland. Clozapine was removed from markets in Europe and never made it to the US, and so Homeland Security (aka the FDA) intervened.
The drug would be re-birthed in 1988 in the United States, in part because of efforts within Homeland Security. The history of Clozapine’s return has been spun and re-spun see The Creation of Psychopharmacology in the course of which a myth has been created that Clozapine is more effective than other antipsychotics even though head to head trials in first episode psychoses show Clozapine to be no better than older drugs like chlorpromazine.
Psychopharmacology
In 2009 the Lancet published a large study by Jouko Tiihonen and colleagues, ironically from Finland, which has thrown the cat among the safety pigeons. In line with expectations, Tiihonen showed that patients treated with antipsychotics had higher mortality rates than patients not on antipsychotics. Except for Clozapine.
Clozapine had a lower mortality rate than all other antipsychotics, lower even than non-treatment.
Did Deniker get it badly wrong?
 So did Deniker get it badly wrong? Have the Lancet redressed an historical error and are they helping Homeland Security by getting American’s on Clozapine or has the Lancet been turned and is it also now a threat to the American people if not to US business?
So did Deniker get it badly wrong? Have the Lancet redressed an historical error and are they helping Homeland Security by getting American’s on Clozapine or has the Lancet been turned and is it also now a threat to the American people if not to US business?
Having spent several posts pointing up the limitations of randomised controlled trials (RCTs), and before going on to labour them further, this Finnish study offers a wonderful example of where RCTs really are needed. The need for an RCT in this case should have been very apparent to the Lancet and its reviewers, and probably should have led to the Tiihonen paper being turned down other than as a marvellous illustration of how badly wrong cross-sectional outcome studies can get things.
Here’s the problem. Outcome studies like the Finnish one are not randomised. The investigators in a study like this just look at what happens in patients taking older antipsychotics like haloperidol, newer drugs like Zyprexa (olanzapine) or Clozapine. Given that thousands of patients may be on each drug and they were followed up over longer periods of time than the usual clinical trial would follow them up, is randomisation we might ask a big deal? Does the accumulation of lots of patients not ultimately manage the bias that randomisation helps us manage? If Clozapine is dangerous could it conceivably show up in a study like this as the safest drug in town?
Outcome studies can get things this badly wrong. Here’s how it can happen. Patients never get Clozapine first line. They have to have taken several other antipsychotics first. This means they will never get Clozapine in their first year of treatment and perhaps not in the first three to five years. The significance of this is that by far the greatest cause of mortality in patients on antipsychotics, over 50% of the mortality, comes from suicide, primarily in the first year of treatment.
The next biggest source of mortality comes from heart attacks and strokes. But these happen for the most part in older patients given antipsychotics for acute and transient psychoses or delusional disorders. These older patients are at some risk of heart attacks or strokes even before treatment. Again these are patients who rarely if ever get Clozapine. There were deaths in the group of patients who were not on any antipsychotic and these most likely came primarily from older patients with acute and transient psychoses.
Add these two exclusions together and it becomes clear why there could be fewer deaths on Clozapine in Finland but how at the same time it can be the most lethal antipsychotic. Clozapine causes problems of unparalleled severity in every body system with deaths from myocarditis, interstitial nephritis, cardiomyopathy, diabetes, neuroleptic malignant syndrome and a range of other causes, including suicide, much more than other antipsychotics, but it is not ordinarily been given to the patients at greatest risk.
But when even the most lethal of the antipsychotics, with a range of cautions like no other, can be billed as the safest, it’s clear we have a system that can induce paranoia. Here’s a question for Danes is the Lancet one of our heroes or has it in some way been turned? A series of Lancet articles touting the benefits of Agomelatin, and the work of Robert Gibbons, and a more general track-record embracing the latest treatments would make anyone wonder.
We used to have a poison sign on most medicines (see We need to talk about doctors). Among antipsychotics Clozapine is the most deserving of such a symbol still.
Source
In Depth
Clozapine is an atypical antipsychotic drug primarily prescribed to patients who are unresponsive to or intolerant of conventional neuroleptics.[13] It is used principally in treating treatment-resistant schizophrenia,[14] a term used for the failure of symptoms to respond satisfactorily to at least two different antipsychotics;[15] It clearly has been shown to be more effective in reducing symptoms of schizophrenia than the older typical antipsychotics, with maximal effects in those who have responded poorly to other medication; though the relapse rate is lower and patient acceptability better, this has not translated to significant observed benefits in global functioning.[14] There is some evidence clozapine may reduce propensity for substance abuse in schizophrenic patients.[16]It is also used for reducing the risk of suicide in patients judged to belong to a high-risk group with chronic risk for suicidal behaviour.
Clozapine was shown to prolong the time to suicidal attempt significantly greater than olanzapine.
Clozapine works well against positive (e.g., delusions, hallucinations) and negative (e.g. emotional and social withdrawal) symptoms of schizophrenia.
It has no dyscognitive effect often seen with other psychoactive drugs and is even able to increase the capabilities of the patient to react to this environment and thereby fosters social rehabilitation. There has been one case report of successful use of clozapine in isolated increase in creatine kinase (in absence of neuroleptic malignant syndrome) in a patient with schizophrenia where other atypical antipsychotics were not successful.[17]
Adverse effects
The use of clozapine is associated with side effects, many of which are minor, though some are serious and potentially fatal: the more common include extreme constipation, bed-wetting, night-time drooling, muscle stiffness, sedation, tremors, orthostatic hypotension, hyperglycemia, and weight gain. The risks of extrapyramidal symptoms such as tardive dyskinesia are much less with clozapine when compared to the typical antipsychotics; this may be due to clozapines anticholinergic effects. Extrapyramidal symptoms may subside somewhat after a person switches from another antipsychotic to clozapine.[18]
Clozapine also carries eleven black box warnings for agranulocytosis, CNS depression, leukopenia, neutropenia, seizure disorder, bone marrow suppression, dementia, hypotension, myocarditis, orthostatic hypotension (with or without syncope) and seizures.[19] Lowering of the seizure threshold may be dose related and slow initial titration of dose may decrease the risk for precipitating seizures. Slow titration of dosing may also decrease the risk for orthostatic hypotension and other adverse cardiovascular side effects.[20]
Clozapine may have a synergistic effect with the sedating action of other drugs such as benzodiazepines, and thus respiratory depression may result with concomitant use. Care should be taken, especially if the latter drugs are given parenterally.
Many male patients have experienced cessation of ejaculation during orgasm as a side effect of clozapine, though this is not documented in official drug guides.[21]
Agranulocytosis
Clozapine carries a black box warning for drug-induced agranulocytosis. Without monitoring, agranulocytosis occurs in about 1% of patients who take clozapine during the first few months of treatment;[22] the risk of developing it is highest about three months into treatment, and decreases substantially thereafter, to less than 0.01% after one year.[23]
Patients who have experienced agranulocytosis with previous treatment of clozapine should not receive it again. In 2007, a pharmacogenetic test was introduced to measure the probability of developing agranulocytosis. The test has two gradations higher and lower risk, with a relative agranulocytosis risk of 2.5 and 0.5 compared to general level. The company states that the test is based on two SNPs of the HLA-DQB1 gene.
Patients taking clozapine are required to have a blood cell count every week, for the first six months of therapy (in the US) and for the first 18 weeks (in the UK). After this, they are required to have a blood cell count every other week for the second six months after therapy. After twelve months, blood cell counts need be performed every four weeks. Patients are advised to inform their doctor if they develop a sore throat, or fever. If the number of white blood-cells drops notably then referral to a haematologist is undertaken.
The manufacturers of both the brand and generic clozapine are required by the FDA to track white blood cells counts for patients receiving clozapine, and pharmacies are required to obtain a copy of the CBC prior to dispensing the medication to the patient. The purpose of the monitoring system is to prevent re-challenge with clozapine in patients with a history of clozapine-induced agranulocytosis and to detect leukopenic events among patients taking clozapine. In other countries (e.g. in Europe), restrictions have been eased.
It has been suggested that coadministration of clozapine with an antioxidant such as vitamin C (ascorbic acid) can reduce the risk of agranulocytosis.[24]
Cardiac toxicity
A more recently identified and sometimes fatal side effect is that of myocarditis, which usually develops within the first month of commencement.[25] First manifestations of illness are fever which may be accompanied by symptoms associated with upper respiratory tract, gastrointestinal or urinary tract infection. Typically C-reactive protein (CRP) increases with the onset of fever and rises in the cardiac enzyme, troponin, occur up to 5 days later. Monitoring guidelines advise checking CRP and troponin at baseline and weekly for the first 4 weeks after clozapine initiation and observing the patient for signs and symptoms of illness.[26]
Signs of cardiac failure are less common and may develop with the rise in troponin. A recent case-control study found that the risk of clozapine-induced myocarditis is increased with increasing rate of clozapine dose titration, increasing age and concomitant sodium valproate.[27] Cardiomyopathy is another potentially fatal cardiac condition that may arise less acutely and may be a result of missed myocarditis. More recently, a regular six-monthly echocardiogram is also recommended to detect cardiomyopathy.
Gastrointestinal hypomotility
Another under recognised and potentially life-threatening side effect spectrum is gastrointestinal hypomotility, which may manifest as severe constipation, fecal impaction, paralytic ileus, bowel obstruction, acute megacolon, ischemia or necrosis. Monitoring of bowel function is recommended, as untreated cases are occasionally fatal.[28][29]
Hyper-salivation
Hyper-salivation (drooling or wet pillow syndrome) is seen in up to 30% of patients on clozapine. While clozapine is a muscarinic antagonist at the M1, M2, M3, and M5 receptors, clozapine is a full agonist at the M4 subset. Because M4 is highly expressed in the salivary gland, its M4 agonist activity is thought to be responsible for the hypersalivation.[30]
Central nervous system
CNS side effects include drowsiness, vertigo, headache, tremor, syncope, sleep disturbances, nightmares, restlessness, akinesia, agitation, seizures, rigidity, akathisia, confusion, fatigue, insomnia, hyperkinesia, weakness, lethargy, ataxia, slurred speech, depression, myoclonic jerks, and anxiety. Rarely seen are delusions, hallucinations, delirium, amnesia, libido increase or decrease, paranoia and irritability, abnormal EEG, worsening of psychosis, paresthesia, status epilepticus, and obsessive compulsive symptoms. Similar to other antipsychotics clozapine rarely has been known to cause neuroleptic malignant syndrome.[31]
Urinary incontinence
Clozapine is linked to urinary incontinence[32] though its appearance may be under-recognised.[33]
Withdrawal effects
Abrupt withdrawal may lead to cholinergic rebound effects, severe movement disorders as well as severe psychotic decompensation. It has been recommended that patients, families, and caregivers are aware of the symptoms and risks of abrupt withdrawal of clozapine. When discontinuing clozapine, gradual dose reduction is recommended to reduce the intensity of withdrawal effects.[34][35]
Weight gain and diabetes
The FDA requires the manufacturers of all atypical antipsychotics to include a warning about the risk of hyperglycemia and diabetes with these medications. Indeed, there are case reports of clozapine-induced hyperglycemia and diabetes. In addition, there are also case reports of clozapine-induced diabetic ketoacidosis.
There is data showing that clozapine can decrease insulin sensitivity. Clozapine should be used with caution in patients who are diagnosed with diabetes or in patients at risk for developing diabetes. All patients receiving clozapine should have their fasting blood glucose monitored.
In addition to hyperglycemia, significant weight gain is frequently experienced by patients treated with clozapine.[36] Impaired glucose metabolism and obesity have been shown to be constituents of the metabolic syndrome and may increase the risk of cardiovascular disease. The data suggest that clozapine may be more likely to cause adverse metabolic effects than some of the other atypical antipsychotics.[37] A study has established that olanzapine and clozapine disturb the metabolism by making the body take preferentially its energy from fat (instead of privileging carbohydrates). Levels of carbohydrates remaining high, the body develops insulin resistance (causing diabetes).[38] Research has indicated that clozapine may cause a deficiency of selenium.[39]
Contraindications
Clozapine is contraindicated in individuals with uncontrolled epilepsy, myeloproliferative disease, or agranulocytosis with prior clozapine treatment. Many other (relative) contraindications (e.g., preexisting cardiovascular or liver damage, epilepsy) also exist.
Interactions
Fluvoxamine inhibits the metabolism of clozapine leading to significantly increased blood levels of clozapine.[40]
Mechanism of action
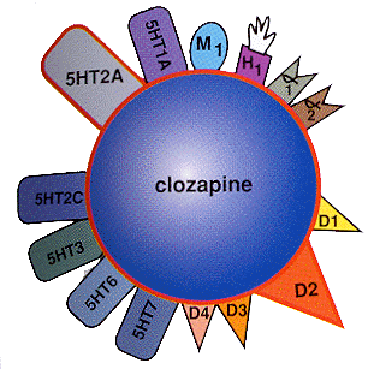
Clozapine is classified as an atypical antipsychotic drug because its profile of binding to serotonergic as well as dopamine receptors;[42] its effects on various dopamine mediated behaviours also differ from those exhibited by more typical antipsychotics.
In particular, clozapine interferes to a lower extent with the binding of dopamine at D1, D2, D3 and D5 receptors, and has a high affinity for the D4 receptor, but it does not induce catalepsy nor inhibit apomorphine-induced stereotypy in animal models as is seen with conventional neuroleptics. This evidence suggests clozapine is preferentially more active at limbic than at striatal dopamine receptors and may explain the relative freedom from extrapyramidal side effects together with strong anticholinergic activity.
Several metabolites of clozapine exhibit binding profiles similar to the original molecule. N-Desmethylclozapine may contribute significantly to the atypical effects of treatment by acting as an agonist and/or partial agonist at D2, D3, δ-opioid, M1, M2, M3, M4, M5 receptors, and an antagonist/inverse agonist at 5-HT2A and 5-HT2C receptors. Clozapine is also a partial agonist at the 5-HT1A receptor, putatively improving depression, anxiety, and the negative cognitive symptoms.[43] Clozapine also is a strong antagonist at different subtypes of adrenergic, cholinergic and histaminergic receptors, the last two being predominantly responsible for its side effect profile.
A direct interaction of clozapine with the GABAB receptor has been shown.[44] GABAB receptor deficient mice exhibit increased extracellular dopamine levels and altered locomotor behaviour equivalent to that in schizophrenia animal models.[45] GABAB receptor agonists and positive allosteric modulators reduce the locomotor changes in these models.[46]
Clozapine induces the release of glutamate and D-serine, an agonist at the glycine site of the NMDA receptor, from astrocytes,[47] and reduces the expression of astrocytic glutamate transporters. These are direct effects that are also present in astrocyte cell cultures not containing neurons. Clozapine prevents impaired NMDA receptor expression caused by NMDA receptor antagonists.[48]
Pharmacokinetics
The absorption of clozapine is almost complete, but the oral bioavailability is only 60 to 70% due to first-pass metabolism. The time to peak concentration after oral dosing is about 2.5 hours, and food does not appear to affect the bioavailability of clozapine. The elimination half-life of clozapine is about 14 hours at steady state conditions (varying with daily dose).
Clozapine is extensively metabolised in the liver, via the cytochrome P450 system, to polar metabolites suitable for elimination in the urine and feces. The major metabolite, norclozapine (desmethyl-clozapine), is pharmacologically active. The cytochrome P450 isoenzyme 1A2 is primarily responsible for clozapine metabolism, but 2C, 2D6, 2E1 and 3A3/4 appear to play roles as well. Agents that induce (e.g., cigarette smoke) or inhibit (e.g., theophylline, ciprofloxacin, fluvoxamine) CYP1A2 may increase or decrease, respectively, the metabolism of clozapine. For example, the induction of metabolism caused by smoking means that smokers require up to double the dose of clozapine compared with non-smokers to achieve an equivalent plasma concentration.[49]
Clozapine and norclozapine plasma levels may also be monitored, though they show a significant degree of variation and are higher in women and increase with age.[50] Monitoring of plasma levels of clozapine and norclozapine has been shown to be useful in assessment of compliance, metabolic status, prevention of toxicity, and in dose optimisation.[49]
Dosage
Due to risk of serious side effects, clozapine treatment is commenced at a very low dose usually 12.5 mg once or twice on the first day[51] and increased slowly until a therapeutic dose is reached.[52][53] In severely ill and/or younger patients higher doses may be needed, while in the elderly much lower doses may be sufficient. Once the patient is stabilised and the maintenance dose has been determined, the greater part or all of the daily dose may be given at bedtime.[53] This will ameliorate daytime sedation and orthostatic problems; most people benefit from the sedation to get to sleep anyway. Furthermore, compliance on medication taken more frequently than once daily drops off dramatically. Norclozapine, the primary metabolite of clozapine, accumulates to, on average, 70% or so of the clozapine concentration in plasma at steady-state (their sample, i.e., pre-dose, ideally in the morning). However, there is substantial variation in the clozapine:norclozapine concentration ratio between individuals.
A steady-state plasma clozapine concentration of 0.35 to 0.6 mg/L (N.B.: quoted values may vary slightly) should produce a clinical response in most patients.
History
Clozapine was developed by Sandoz in 1961,(see above and 1958 under Wander) and trials took place in 1972, when it was released in Switzerland and Austria as Leponex. Two years later it was released in West Germany, and Finland in 1975. Early testing was performed in the United States around the same time.[54] In 1975, after reports of agranulocytosis leading to death in some clozapine-treated patients, clozapine was voluntarily withdrawn by the manufacturer.[55] Clozapine fell out of favour for more than a decade. However, when studies demonstrated that clozapine was more effective against treatment-resistant schizophrenia than other antipsychotics, the FDA and health authorities in most other countries approved its use only for treatment-resistant schizophrenia, and required regular haematological monitoring to detect granulocytopenia, before agranulocytosis develops. In December 2002, clozapine was approved in the US for reducing the risk of suicide in schizophrenic or schizoaffective patients judged to be at chronic risk for suicidal behaviour.[56] In 2005 FDA approved criteria to allow reduced blood monitoring frequency.[57]
Notes
[1] Hopfinger A, Esposito EX, Llinas A, Glen RC, Goodman JM.. Findings of the Challenge To Predict Aqueous Solubility. Journal of Chemical Information and Modeling. 2009;49:1-5.
[2] Wyatt RJ; Chew RH (2005). Wyatt’s Practical Psychiatric Practice: Forms And Protocols For Clinical Use. American Psychiatric Association. pp. 39.
[3] Kane, J; Honigfeld G, Singer J, Meltzer H (September 1988). Clozapine for the treatment-resistant schizophrenic: a double-blind comparison versus chlorpromazine/benztropine. Archives of General Psychiatry 45 (9): 789796.
[4]From Clozapine Package Insert: Frequency of Monitoring based on Stage of Therapy or Results from WBC Count and ANC Monitoring Tests (pdf). TEVA Clozapine Patient Registry. Retrieved 2012-08-18.
[5] Wallis C & Willwerth J (July 6, 1992). Awakenings Schizophrenia a New Drug Brings Patients Back to Life. Time. Retrieved 2008-10-09.
[6] Clozaril (Clozapine) drug description FDA approved labelling for prescription drugs and medications at RxList Rxlist.com. Retrieved 2008-10-09.
[7] National Institute of Mental Health.What medications are used to treat schizophrenia? Retrieved 2012-08-18.
[8] From Clozapine Package Insert: Frequency of Monitoring based on Stage of Therapy or Results from WBC Count and ANC Monitoring Tests (pdf). TEVA Clozapine Patient Registry. Retrieved 2012-08-18.
[9] Townsend, G.; Curtis, D. (2006). Case report: Rapidly fatal bowel ischaemia on clozapine treatment. BMC Psychiatry 6: 43.
[10] Devinsky, O.; Honigfeld, G.; Patin, J. (1991). Clozapine-related seizures. Neurology 41 (3): 369371. PMID 2006003.
[11] De Berardis, D.; Serroni, N.; Campanella, D.; Olivieri, L.; Ferri, F.; Carano, A.; Cavuto, M.; Martinotti, G. et al. (2012). Update on the adverse effects of clozapine: Focus on myocarditis. Current drug safety 7 (1): 5562.
[12] Hartling, L.; Abou-Setta, A. M.; Dursun, S.; Mousavi, S. S.; Pasichnyk, D.; Newton, A. S. (2012). Antipsychotics in Adults with Schizophrenia: Comparative Effectiveness of First-Generation Versus Second-Generation Medications: A Systematic Review and Meta-analysis Annals of internal medicine.
[13] MIMS Ireland. April 2007.
[14] Wahlbeck K, Cheine MV, Essali A (2007). Wahlbeck, Kristian. ed. Clozapine versus typical neuroleptic medication for schizophrenia. The Cochrane Database of Systematic Reviews (John Wiley and Sons, Ltd.) (2): CD000059.
[15] Meltzer HY (1997). Treatment-resistant schizophrenia the role of clozapine. Current Medical Research and Opinion 14 (1): 120.
[16] Lee M, Dickson RA, Campbell M, Oliphant J, Gretton H, Dalby JT.. Clozapine and substance abuse in patients with schizophrenia. Canadian Journal of Psychiatry. 1998;43:855856.
[17] P03-358 Clozapine may be the answer: a case report of elevated serum creatine kinase in the absence of NMS. A. Mohandas, N. Talwar, A. James Langdon Hospital, Devon Partnership NHS Trust, Dawlish, UK
[18] Clozapine.
[19] http://www.clinicalpharmacology-ip.com/Forms/Monograph/monograph.aspx?cpnum=142&sec=monadve
[20] Clozapine.
[21] Baggaley, M. (2008). Sexual dysfunction in schizophrenia: Focus on recent evidence. Human Psychopharmacology: Clinical and Experimental 23 (3): 201209.
[22] Baldessarini, Ross J.; Frank I. Tarazi (2006). Pharmacotherapy of Psychosis and Maa. In Laurence Brunton, John Lazo, Keith Parker (eds.). Goodman & Gilmans The Pharmacological Basis of Therapeutics (11th ed.). New York: McGraw-Hill.
[23 Alvir JM, Lieberman JA, Safferman AZ, Schwimmer JL, Schaaf JA (1993). “Clozapine-induced agranulocytosis. Incidence and risk factors in the United States”. N Engl J Med 329 (3): 1627. Free full text with registration
[24] Hsyuanyu Y., Dunford H.B. (1999). Oxidation of Clozapine and Ascorbate by Myeloperoxidase. Archives of Biochemistry and Biophysics 368 (2): 412420.
[25] Haas SJ, Hill R, Krum H (2007). Drug Safety 30 (1): 4757.
[26] Ronaldson KJ, Taylor AJ, Fitzgerald PB, Topliss DJ, McNeil JJ. (2011). A new monitoring protocol for clozapine-induced myocarditis based on an analysis of 75 cases and 94 controls.. Aust NZ J Psych 45: 458465.
[27] Ronaldson KJ, Fitzgerald PB, Taylor DJ, Topliss DJ, Wolfe R, McNeil JJ. (2012). Rapid clozapine dose titration and concomitant sodium valproate increase the risk of myocarditis with clozapine: A case-control study. Schizophr Res 141: 173-8.
[28] Palmer SE, McLean RM, Ellis PM, Harrison-Woolrych M (2008). Life-threatening clozapine-induced gastrointestinal hypomotility: an analysis of 102 cases. Journal of Clinical Psychiatry 69 (5): 759768.
[29] Townsend G, Curtis D (2006). Case report: rapidly fatal bowel ischaemia on clozapine treatment. BMC Psychiatry 6: 43.
[30] http://www.medscape.com/viewarticle/409612_2
[31] rxlist.com / Clozapine side effects
[32] Raja, M. (2011). Clozapine safety, 35 years later. Current drug safety 6 (3): 164184.
[33] Barnes, T. R. E.; Drake, M. J.; Paton, C. (2012). Nocturnal enuresis with antipsychotic medication. The British Journal of Psychiatry 200 (1): 79. d
34] Ahmed, S.; Chengappa, KN.; Naidu, VR.; Baker, RW.; Parepally, H.; Schooler, NR. (Sep 1998). Clozapine withdrawal-emergent dystonias and dyskinesias: a case series. J Clin Psychiatry 59 (9): 4727.
[35 Szafra„ski, T.; Gmurkowski, K. (1999). “[Clozapine withdrawal. A review]. Psychiatr Pol 33 (1): 5167.
[36] Wirshing DA, Wirshing WC, Kysar L, Berisford MA (1999). Novel antipsychotics: comparison of weight gain liabilities. Journal of Clinical Psychology 60 (6): 35863.
[37] Nasrallah HA (January 2008). Atypical antipsychotic-induced metabolic side effects: insights from receptor-binding profiles. Mol. Psychiatry 13 (1): 2735.
[38] Albaugh VL; Vary TC; Ilkayeva O; Wener BR; Maresca KP; Joyal JL; Breazeale S; Elich TD et al. (2010). Atypical Antipsychotics Rapidly and Inappropriately Switch Peripheral Fuel Utilization to Lipids, Impairing Metabolic Flexibility in Rodents Schizophrenia Bulletin 38 (1): 15366.
[39] Vaddadi KS, Soosai E, Vaddadi G (2003). Low blood selenium concentrations in schizophrenic patients on clozapine. British Journal of Clinical Pharmacology 55 (3): 3079.
[40] Sproule B. A., Naranjo C. A., Brenmer K. E., Hassan P. C. (December 1997). Selective serotonin reuptake inhibitors and CNS drug interactions. A critical review of the evidence. Clin Pharmacokinet 33 (6): 45471.
[41] Novartis Pharmaceuticals (April 2006).Prescribing Information (PDF). Novartis Pharmaceuticals. pp. 36. Retrieved 2007-06-29.
[42] Naheed M, Green B. (2001). Focus on clozapine. Curr Med Res Opin 17 (3): 2239.
[43] Robinson DS (2007). CNS Receptor Partial Agonists: A New Approach to Drug Discovery. Primary Psychiatry 14 (8): 2224.
[44] Wu Y, Blichowski M, Daskalakis ZJ, Wu Z, Liu CC, Cortez MA, Snead OC 3rd. (2011). Evidence that clozapine directly interacts on the GABAB receptor. Neuroreport. 22 (13): 63741.
[45] Vacher CM, Gassmann M, Desrayaud S, Challet E, Bradaia A, Hoyer D, Waldmeier P, Kaupmann K, Pvet P, Bettler B. (2006). Hyperdopaminergia and altered locomotor activity in GABAB1-deficient mice. J Neurochem. 97 (4): 97991.
[46] Wiero„ska JM, Kusek M, Tokarski K, Wabno J, Froestl W, Pilc A. (2011). The GABA B receptor agonist CGP44532 and the positive modulator GS39783 reverse some behavioural changes related to positive syndromes of psychosis in mice Br J Pharmacol. 163 (5): 103447.
[47] Tanahashi S, Yamamura S, Nakagawa M, Motomura E, Okada M. (2012). Clozapine, but not haloperidol, enhances glial D-serine and L-glutamate release in rat frontal cortex and primary cultured astrocytes. Br J Pharmacol. 165 (5): 154355.
[48] Xi D, Li YC, Snyder MA, Gao RY, Adelman AE, Zhang W, Shumsky JS, Gao WJ. (2011). Group II metabotropic glutamate receptor agonist ameliorates MK801-induced dysfunction of NMDA receptors via the Akt/GSK-3β pathway in adult rat prefrontal cortex. Neuropsychopharmacology. 36 (6): 126074.
[49] Rostami-Hodjegan A, Amin AM, Spencer EP, Lennard MS, Tucker GT, Flanagan RJ (2004). Influence of dose, cigarette smoking, age, sex, and metabolic activity on plasma clozapine concentrations: a predictive model and nomograms to aid clozapine dose adjustment and to assess compliance in individual patients J Clin Psychopharmacol 24 (1): 708.
[50] Lane HY, Chang YC, Chang WH, Lin SK, Tseng YT, Jann MW. (January 1999). Effects of gender and age on plasma levels of clozapine and its metabolites: analysed by critical statistics J Clin Psychiatry 60 (1): 3640.
[51] Healy 2009,pg 19 Psychiatric drugs explained 5th Ed.Edinburgh : Elsevier Churchill Livingstone
[52] Novartis Pharmaceuticals.Clozaril Dosing Guide. Novartis Pharmaceuticals. Retrieved 2007-06-29.
[53] 4.2.1 Antipsychotic drugs British National Formulary (55 ed.). March 2008. pp. 195.
[54] Crilly, John (2007). The history of clozapine and its emergence in the US market: a review and analysis History of Psychiatry 18 (1): 3960.
[55] Healy, David (2004). The Creation of Psychopharmacology. Cambridge: Harvard University Press. pp. 23842.
[56] Supplimental NDA Approval Letter for Clozaril, NDA 19-758 / S-047. United States Food and Drug Administration. December 18, 2002. Archived from the original on November 23, 1012. Retrieved November 23, 2012.
[57]http://www.accessdata.fda.gov/drugsatfda_docs/appletter/2005/019758s054ltr.pdf
Further Study
DHA-clozapine
Drug of last resort
Medicating for the slave mentality : Society of Behavioural MedicineÂ
Cranial manipulation and the bone setters
The pineal gland, electromagnetic fields, ELF and chemistry
Froebel Education Method and the Third Reich
Tags : Antipsychotic, Azaleptin, Behaviour Change, Clozapine, Clozaril, Denzapine, Fazaclo, Froidir, Klazapol, Lancet, Leponex, Neuroleptic, NICE, Pierre Deniker, Pineal Gland, Sandoz Pharmaceuticals, Wander, Zaponex
